Abstract
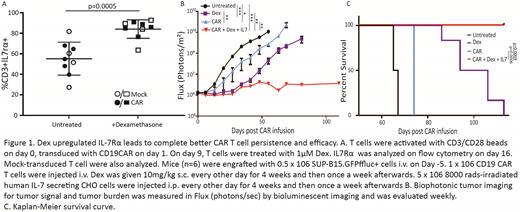
Dexamethasone (Dex) has been a mainstay for the treatment of inflammatory pathologies, such as in autoimmunity and cytokine release syndrome (CRS) from immunotherapies. However, its effects on chimeric antigen receptor (CAR) T cells, during CRS events have not been interrogated. In this study, we treated the CAR T cells with different concentrations of Dex (0, 1, and 10µM) single or multiple times in vitro and expanded them for one week. We demonstrated that Dex treatment did not inhibit CAR T cell growth and functionality even with concentrations higher than what would be used in the clinic. Interestingly, we observed that the Dex treatment significantly upregulated endogenous gamma chain cytokine receptor-interleukin 7 receptor alpha (IL7Rα) at the mRNA and protein levels (P=0.0005) (Fig 1a). These effects are not T cell subset dependent because we observed upregulation of IL7Rα on PBMC and enriched naïve and memory T cell-derived CAR T cells. Furthermore, un-transduced T cells also exhibited IL7Rα increase, which suggests that the upregulation of IL7Rα is the general mechanism of Dex for T cells. IL7Rα is well accepted as a key element to CAR T cell persistence and memory T cell formation. However, the IL7R-IL-7 signaling pathway is limited due to the downregulation of high-affinity IL7Rα during the activation and expansion of CAR T cells. We found out that Dex can upregulate endogenous IL7Rα in a reversible manner, which is an important factor for safety in clinical application. We showed that ex vivo upregulation of IL7Ra by a single Dex treatment subsequently enhanced CAR T cell persistence and anti-tumor efficacy in vivo in the presence of IL-7. To further confirm the positive effects of Dex on CAR T cell therapy, we performed a combinatorial therapy by delivering CD19 CAR T cells to acute lymphoid leukemia (ALL) tumor-bearing NOD-scid IL2Rgammanull (NSG) mice and then administering Dex (1mg/kg) and IL-7-expressing CHO cells. Consistently, we observed a complete cure of tumor-bearing mice only in the CD19 CAR T cell group that was given both Dex and IL-7, but not CAR alone and Dex only groups (Fig 1b-c) (P=0.0006). Mice survived, tumor-free, over 150 days. Supportively, we observed CAR T cell persistence only in the CAR T cells combined with Dex and IL7 group but not in the CAR group. To determine if Dex influenced CAR T cells beyond IL7Rα, we performed gene analysis and demonstrated that IL7Rα, but not other γ chain cytokines was selectively upregulated by Dex, which supports previous reports from Lee et al. that Dex and glucocorticoid receptors (GR) complex binds upstream of the IL-7rα promoter preferentially regulating IL7Rα. Furthermore, we utilized Nanostring technology to analyze CAR T cells with and without Dex treatment for mRNA signatures that related to signaling pathways. We observed pathways related to activation, migration, persistence, and chemokine production were upregulated, while pathways related to apoptosis and TCR diversity were downregulated after Dex treatment. The results indicated that Dex may regulate multiple functions of CAR T cells.
Overall, our studies in both in vitro and in vivo treatment support that Dex does not have negative effects on CAR T cell potency but provides insight into an unforeseen strategy to improve CAR T cell therapies through upregulation of IL-7Rα and improving T cell activation, trafficking, and persistence. We believe our observations could extend beyond hematological malignancies to a potentially potent and durable therapy for solid tumors, as Dex is not only an immunosuppressive agent but also an anti-cancer drug used against a multitude of tumors to prevent tumor growth as well as modulate the microenvironment. Our data also provided rationale on starting CAR T cell therapy without the necessity of tapering off the ongoing steroid treatment.
Forman: Allogene: Consultancy; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company. Wang: Pepromene Bio, Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal